Background: Non-transfusion-dependent thalassemia (NTDT) characterized by ineffective hematopoiesis and increased intestinal iron absorption, and excessive iron deposits in the bone marrow, heart, and liver, causing progressive organ damage. It has been reported that oxidative stress damage caused by iron overload could induce apoptosis or ferroptosis. However, the related studies and mechanisms are not well unknown in NTDT disorders.
Aims: To investigate the effects of iron overload on bone marrow erythropoiesis, heart and liver in NTDT patients and Hbb th3(th3/+) mice.
Methods: Patients (19-47 y of age) and 12-month-old Hbb th3(th3/+) mice with NTDT were selected as thalassemia group, healthy human and normal mice were selected as control group. Iron overload related indexes, bone marrow erythropoiesis, oxidative stress, apoptosis and ferroptosis of each sample were detected by Elisa, flow cytometry, RT-PCR and Weston blot methods, respectively.
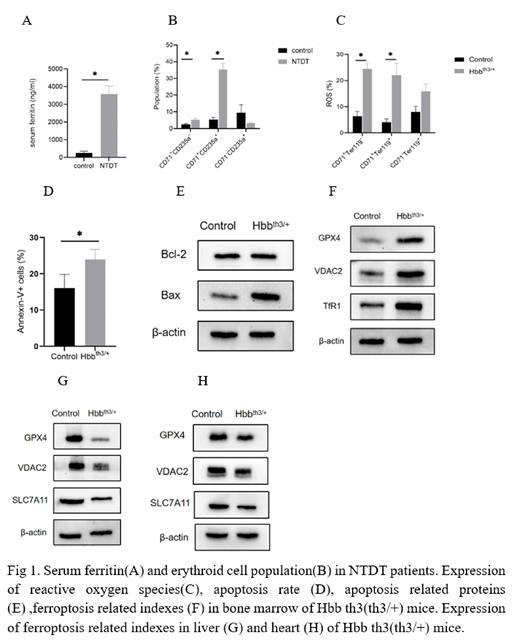
Results: Compared with the control group, Serum ferritin is significantly increased in NTDT group, and there is imbalance of iron metabolism. And in NTDT patients, liver and myocardial iron deposition was assessed by MRI analysis, resonance results suggest that the degree of iron overload in liver is more significant than that in heart. The damage of erythropoiesis in NTDT group was more severe than that in control group, which showed active erythropoiesis, and the differentiation of erythroid precursor cells stagnated in early stage of erythroid precursor cells, and there were maturation disorders. The oxidative stress-related indexes reactive oxygen species (ROS), lipid peroxidation and malondialdehyde (MDA) induced by iron overload were also significantly increased in the NTDT group. However, significant signs of apoptosis were observed in the bone marrow of the NTDT group, including increased proportion of apoptotic cells, signs of apoptosis were mainly observed in the mitochondria of erythroid colony electron microscopy, and the level of GPX4 that is a key indicator of ferroptosis, was increased. But the expression of GPX4, VDAC2 and SLC7A11 of ferroptosis was decreased in the heart and liver with heavy iron deposition, and the signs of ferroptosis were also observed under electron microscopy.
Summary/Conclusion: Our study found that there are differences in iron overload and injury performance among different organs in NTDT. Oxidative stress caused by iron overload in the bone marrow impairs erythropoiesis mainly through apoptosis, whereas signs of ferroptosis were observed in the liver and heart of Hbb th3(th3/+) mice.These results suggest that with the gradual accumulation of iron circulation, the damage is a dynamic process, and different organs show different signs of damage, which may be related to the different mechanisms of damage, tolerance and compensation of organs, but further studies are needed to confirm.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal